Are hydrocooling and fruit washing critical control points (CCPs)?
Determining whether or not hydrocooling and fruit washing constitute a critical control point starts with your hazard analysis and risk assessment. Within that analysis and assessment, and according to PrimusGFS 3.2, 6.02.01 interpretation guidelines: one should “Consider pre-requisite programs (PRPs) in place which provide basic environmental and operating conditions necessary for the production of safe, wholesome food and support decisions in the hazard analysis.” And in 6.02.02: “The CCPs should be created from the documented hazard analysis i.e. there should be a logical documented approach (such as utilizing a CCP decision tree that justifies whether or not there is a step(s) in the process determined to be a CCP(s).”
In CODEX General Principles of Food Hygiene CXC 1-1969, Revised 2022, you will find “Figure 1: Example of a CCP decision tree,” and this question:
A recent CONTACT webinar on washwater included an excellent discussion of this topic led by Faith Critzer, Ph.D. from the University of Georgia. (A recording of that particular webinar should be posted by mid-May, but there are plenty of others on that site worth your time. Unfortunately, the future of this webinar series is in jeopardy as it depends on grant funds from the USDA Specialty Crop Research Initiative.)
In a recent, personal communication, Dr. Critzer said:
“If your current program is being properly implemented and you have records to show this (water quality parameters, sanitizer concentration) and microbiological evidence through on-site analysis to show that produce exiting the water system has on average lower or similar populations of indicators or aerobic plate counts than on entry, you would have sufficient evidence and justification that your water management prerequisite program is properly operating to manage the hazard, thereby reducing its likelihood of occurrence. On the other hand, if your records show that you’re regularly not meeting your operational limits for various parameters or if the program isn’t being properly monitored, this will increase the likelihood of cross-contamination, and it should be elevated to a CCP.”
There is sufficient evidence in the research literature to guide decisions on the water quality parameters and sanitizer concentration necessary to manage hazards during hydrocooling and fruit washing to an acceptable level within the context of your prerequisite programs and thereby justify the decision that they are not CCPs as long as all other elements described by Dr. Critzer above are in place.
Many of those references can be found on this site under “Resources > Hydrocool & Wash Water.”
Please don’t hesitate to contact me if you have any questions on this topic.
Should you rotate sanitizers?
In discussing which sanitizers are best, the subjects of pathogen resistance and the need to rotate sanitizers often come up. Suggesting that sanitizer rotation is not necessary is often met with some level of push-back or incredulity.
This subject is not only relevant to optimizing one’s sanitation program, but it is also a good example of why we should always exercise a healthy dose of skepticism and test our assumptions.
As a starting point, it is certainly reasonable to think that sanitizer rotation should be a consideration, but what does the evidence say? The best evidence we have so far indicates that the level of sanitizer resistance tolerance exhibited by pathogens of greatest concern, Listeria monocytogenes, E. coli, and Salmonella, does not warrant sanitizer rotation when products are used at manufacturer-recommended concentrations (MRCs).
If you disagree, it would be great to hear from you. Don’t forget to bring your evidence. 😉
Probing antimicrobial resistance and sanitizer tolerance themes and their implications for the food industry through the Listeria monocytogenes lens
Bland, R., Brown, S. R. B., Waite-Cusic, J., & Kovacevic, J. (2022). Compr Rev Food Sci Food Saf, 21, 1777–1802.
Sanitizer rotation is a practice where sanitizers with different active ingredients are cycled in and out of use on a predetermined schedule, with the underlying intention of reducing the likelihood of the facility microbiome developing AMR. The practice of sanitizer rotation is frequently proposed by a number of book chapters, articles, publications, and guidance documents, but its necessity is debated (Bodnaruk, 2011; Cutter, 2003; FDA, 2017; Marriott et al., 2018; Møretrø et al., 2017; Tompkin et al., 1999; USDA FSIS, 2015). The underlying rationale behind sanitizer rotation is based on antibiotic resistance research, specifically the potential mutability and acquisition of genetic elements to support tolerance/resistance and/or the ability to modify gene expression under selective pressures by altering cell wall and membrane permeability or by increasing the expression of efflux pumps (Noll et al., 2020).
The practice of sanitizer rotation is also mentioned in both FDA's draft guidance for the control of L. monocytogenes in RTE foods (FDA, 2017) and FSIS best practices guidance for controlling L. monocytogenes in retail delicatessens (USDA FSIS, 2015). Both of these documents state that rotating sanitizers will provide greater effectiveness in the long term and prevent the establishment of L. monocytogenes in the processing environment (FDA, 2017; USDA FSIS, 2015). However, the references provided to support these statements are either guidelines or extension articles that share observations of common industry practices (Cutter, 2003; Tompkin et al., 1999), without scientific data.
Read full article HERE
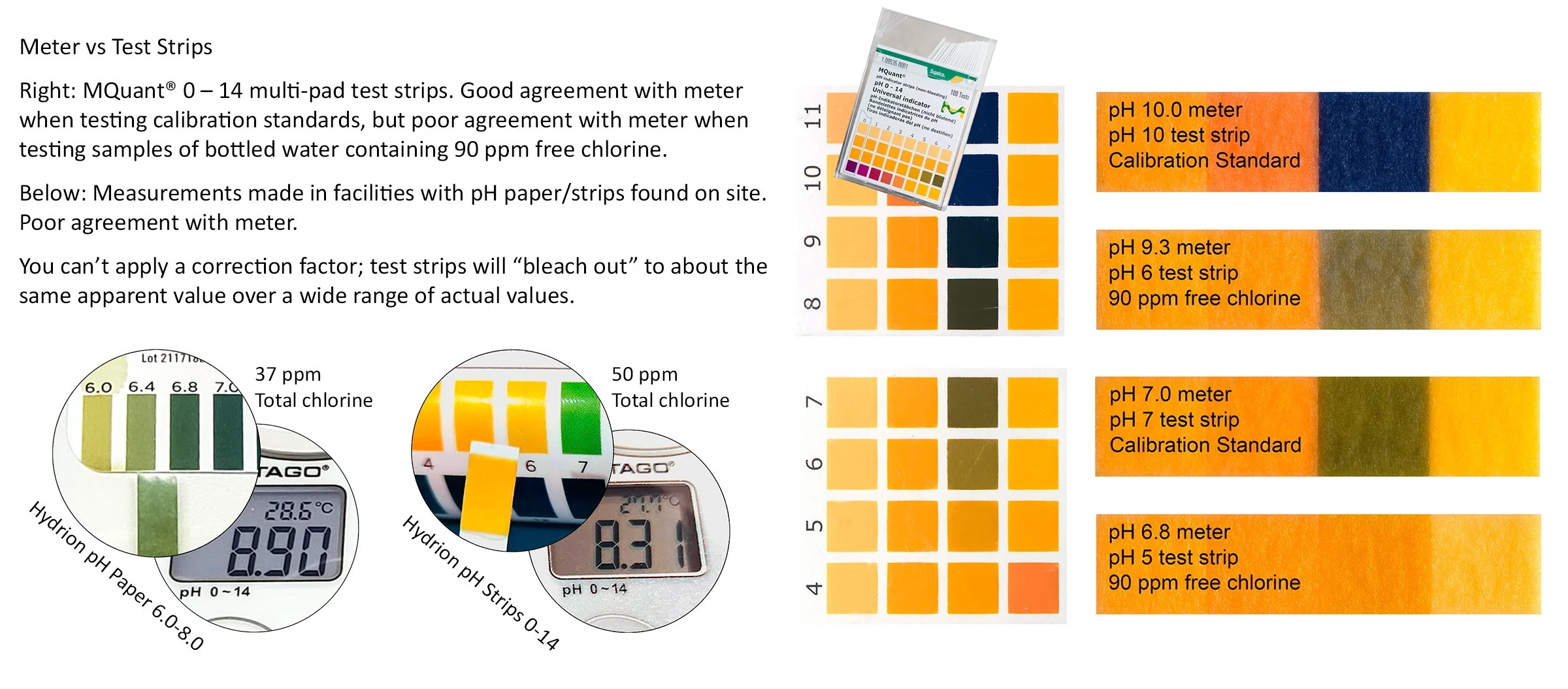
Throw away your pH test strips now!
Accurate monitoring and control of pH is critical for getting the most sanitizing “power” and bang for your buck out of sodium hypochlorite (chlorine bleach) solutions.
Test strips may be “accurate enough” when measuring the pH of drinking water or pool water containing very low concentrations of chlorine (less than 5 ppm). However, the concentration of sodium hypochlorite used in hydrocoolers and wash systems is usually great enough to cause chlorine to react with test strip dyes and change their color response to produce a much lower apparent pH value compared to actual. You may think the pH is 7, or even less, when it is actually 8 or 9.
Incorrect measurements may be worse than no measurements at all because they can give one a false sense that everything is okay when it is not. More than once, I have seen test strip results used as an argument for not controlling pH with an acidifying agent when actual pH was clearly too high as measured with an accurate, properly calibrated meter.
Sodium hypochlorite decreases from 80% active ingredient to 30% active ingredient as pH increases from 7 to 8. At pH 8, that’s like paying $13/gal instead of $5/gal for bleach. Good monitoring and control of pH pays for itself.
For most systems, a pH range of 6.5-7.5 on paper should accommodate normal system variation, but to the extent you can, it is best to maintain the pH of sodium hypochlorite solutions as close to 6.8-7.0 as possible.
NEVER USE TEST STRIPS TO MEASURE THE pH OF CHLORINATED HYDROCOOL OR WASH WATER.
Fund the science, find solutions, fuel the change
It’s important to recognize that a clean test result doesn’t confirm that your food is safe, rather it verifies that your cleaning processes are effective.
The two-day 2023 CPS Research Symposium wrapped up this past Wednesday in Atlanta. Focus was on how to translate the latest food safety learnings into action: “Fuel the Change”
The Center for Produce Safety (CPS) is a 501(c)(3), U.S. tax-exempt, charitable organization focused exclusively on providing the produce industry and government with open access to the actionable information needed to continually enhance the safety of produce.
The total value of CPS-funded, just-completed and in-progress research represented here, which is relevant to CFFA members, is 5 million dollars. Previous projects of interest to CFFA members worth several million more date back to 2015.
FINAL REPORTS
Paul Dawson, PhD, from Clemson University demonstrated that Listeria monocytogenes and Salmonella biofilms could survive on plastic surfaces for over two weeks under dry conditions. (CFFA collaboration) During 2022, food contact surfaces at two stone fruit packing facilities were swabbed before and after sanitation over the course of several weeks. It was revealed that typical sanitation practices were least effective on brush beds and sizer carriers. Following Dr. Dawson’s presentation during a discussion with attendees from industry, led by Dr. Trevor Suslow, it was emphasized that sanitation soon after operations end is necessary to minimize microbial load and risk. For instance, if a packline is to remain idle for any length of time, it is best to do a deep clean (with disassembly as necessary) immediately after the last run and follow up with another “regular” sanitation just before operations are restarted. In a sidebar discussion with Dr. Martin Wiedmann of Cornell University, he emphasized the importance of disassembling components of high-risk equipment like washer-waxers to eliminate persistent Listeria biofilms. (Note: Listeria monocytogenes is not the only pathogen that may be associated with biofilms. Pathogens in general often exist in complex microbial communities and may be found within biofilms regardless of their own ability to form a biofilm. For instance, while Salmonella may not persist and spread throughout a facility over time like Listeria monocytogenes, its association with biofilms can help it survive longer and resist sanitation efforts more than if it were on its own. Bottom line: Elbow grease is essential, don’t count on sanitizers to do their job unless the surface is clean.)
Matthew Stasiewicz, PhD, from the University of Illinois at Urbana-Champaign reported on his work modeling product testing and concluded: Effective interventions reduce contamination and make pathogens harder to detect. Therefore, testing is best for detecting incoming, high-level contamination, or otherwise unexpected or uncontrolled sources of contamination, and of limited value for finished product. These results reinforce the well-established principle that focus must be on production and handling processes and food safety interventions rather than finished product testing to achieve the highest levels of food safety.
Nitin Nitin, PhD, from UC Davis presented his final report on a project to evaluate the durability and efficacy of antimicrobial coatings. (CFFA collaboration) “Food-grade biopolymers charged with chlorine can form flexible antimicrobial coatings on diverse food-handling surfaces to reduce cross-contamination of fresh produce with foodborne pathogens.” Research included testing on stainless steel stone fruit packing tables. These coatings are compatible with a wide variety of surfaces and equipment.
Disinfection of washwater in apple dump tanks. Meijun Zhu, Washington State University.
The waxing of whole produce (citrus) and its impact on microbial food safety. Luxin Wang, UC Davis.
Evaluating the challenges presented by blueberry harvest equipment sanitation. Jinru Chen, University of Georgia.
Understanding and predicting food safety risks posed by wild birds, Eastern US. Nikki Shariat, University of Georgia.
Environmental monitoring and patterns of Listeria contamination in fresh produce processing environments. Ana Allende, CEBAS-CSIC (Spain)
RESEARCH IN PROGRESS
Evaluate performance of common washwater sanitizers for the control of Salmonella and Listeria monocytogenes on peaches under laboratory and commercial conditions. Meijun Zhu, Washington State University. (CFFA collaboration)
Addition of antimicrobials to fruit waxes to enhance food safety and extend shelf life. Oixin Zhong, University of Tennessee. (CFFA collaboration)
Rapid, user-friendly sampling and test method as a tool to assess risks associated with adjacent cattle operations. Mohit Verma, Purdue University. (CFFA collaboration)
Cross-contamination risks in dry environments. Nitin Nitin, UC Davis. (CFFA collaboration)
Practical application of superheated steam to harvesting, processing, and produce packing equipment. Abby Snyder, Cornell University.
Effective strategies to sanitize harvest bins and picking bags. Valentina Trinetta, University of Georgia.
Assessment of the food-safety risks imposed by wild birds, Western US. Daniel Karp, UC Davis.
Interaction of microbial communities and Listeria on pears during cold storage. Meijun Zhu, University of Washington.
Understanding potential differences between intact and wounded fruit on microbial communities and interactions with Listeria on pears in the long-term storage environment. Laura Strawn, Virginia Tech.
For details of these projects, please visit the CPS website or if you have questions or would like to discuss, head on over to the forum. If you visit the CPS website for details, use the name of the research scientist for your search to find the project.
The only thing that matters is everything
It’s important to recognize that a clean test result doesn’t confirm that your food is safe, rather it verifies that your cleaning processes are effective.
Because we cannot take just one big step (kill step) to get us where we want to go, we must take many small steps all heading in the same direction to produce safe fresh fruit.
When I talk to folks about effective sanitation, lots of details come up: what kind of rag is best, what kind of cleaning methods should be avoided, the principle that you can’t sanitize a dirty surface, clean from top down, and many more.
Sometimes, I will get a little pushback like: “Seriously, does it really matter what kind of rag I use?” Probably not a lot, but why wouldn’t one at least consider using the best available option? There is some evidence that microfiber towels are superior to common terrycloth bar towels or cotton shop towels for both removing pathogens from surfaces and not transferring pathogens back to surfaces (1). The difference is not large, it’s not going to transform your sanitation program from average to excellent, but choosing the best tools for sanitation is not a trivial matter.
When it comes to tree fruit, a more significant detail to consider is washwater management. Contact time, sanitizer selection and concentration, and in the case of sodium hypochlorite, pH control are important variables that affect how well your fruit wash performs in reducing microbiological load. (I’ll leave out the effects of temperature, addition of soap, and mechanical scrubbing action for now.)
Most of us would agree that our wash systems would benefit from increased contact time, but doing much about that is usually a very expensive proposition and not something you are going to run out and change after reading this. What can we reasonably do right now? In the case of sodium hypochlorite, we can optimize concentration and pH. Research indicates that a total chlorine concentration of around 25 ppm at a “good” pH of 7.0 is not much better, if any better at all, than a freshwater rinse without sanitizer in reducing the microbial load on incoming fruit. Antimicrobial performance improves as concentration increases to 100 ppm (2, 3), and I think it is likely that dose response continues up to at least 150 ppm (personal communication, research in progress).
The reason I am given most often from those who favor low total chlorine concentration (<75 ppm) and the addition of little or no acidifying agent (pH >7.0) in stone fruit washwater is concern about fruit injury, usually staining. Staining is a type of skin discoloration that starts with abrasion injury. No abrasion injury, no staining. It has been reported that “pHs over 8.0‐8.5 induced dark staining (burning) in the different cultivars tested. Extremely darker skin staining was observed after the fruit was exposed to pH 9.0, 9.5 and 10.0 solutions in susceptible cultivars” (4). Add sodium hypochlorite, which increases pH, and don’t add acidifying agent to decrease pH, and what do you get? Increased staining potential and decreased antimicrobial activity.
In a nutshell, after 20+ years of following this issue, I have never seen stone fruit injury caused by properly-managed, single-pass wash systems using sodium hypochlorite at 100-120 ppm total chlorine at pH 6.8-7.0. Don’t miss the opportunity to reduce food safety risk by optimizing fruit wash parameters.
So, there’s two examples of details that matter. Obviously, one is more likely to have a significant effect than the other, but nevertheless, every detail, every step you take counts. Do what you can to take as many steps in the direction of reducing food safety risk as possible.
Environmental monitoring
It’s important to recognize that a clean test result doesn’t confirm that your food is safe, rather it verifies that your cleaning processes are effective.
It’s important to recognize that a clean environmental monitoring test result doesn’t confirm that your food is safe, rather it verifies that your cleaning processes are effective.
Sanitation verification by ATP meter or aerobic plate count (APC) answers the question: Did I do what I said I was going to do? Verification is not the same thing as environmental monitoring. Environmental monitoring (EM) answers the question: Is what I said I was going to do in my SSOP good enough?
A strong EM program can also help limit the scope of a potential recall, or even prevent a recall by providing evidence of an effective sanitation program and corrective actions, when necessary.
Do not implement an EM program until after you have thought through and documented a corrective action process, should it become necessary.
The most likely reason that an EM program never detects Listeria species is because “the sampling and/or testing procedures are not rigorous or sensitive enough.” Listeria monocytogenes (LM) is the pathogen of greatest concern for most fruit packing facilities, and it is also a natural inhabitant of the growing environment, so it is likely to be present at some level at some time in all packing facilities.
“Being so close to product contact surfaces, they [Zone 2 surfaces] are more likely than Zone 3 to accumulate moisture and nutrients and, if Listeria become entrenched, provide a shorter distance to product contact surfaces. Detection of Listeria on a Zone 2 surface should be taken seriously; since Zone 2 is not product contact, any Listeria detected are less likely to be transients from incoming produce and may be more likely coming from the production environment itself.”
Ten sites and five samples from among those ten sites every two weeks for Listeria species should be considered the minimum for even the smallest of operations. In other words, identify a total of ten sampling sites and then sample five of those sites one week, followed by sampling the other five sites two weeks later; continue to alternate sites in that way every two weeks throughout the season. Focus on Zone 2 and wherever moisture and exposed product are present together, for instance, washer-waxer framework. Make sure highest-risk areas are represented in each set of biweekly samples. Sample a similar number of food contact surface sites every two weeks for APC or Enterobacteriaceae (EB), depending on risk assessment.
Facilities with hydrocoolers and cold storages include additional challenges involving LM due to the combination of moisture and traffic. While LM on a wet cold-room floor may have little chance of contaminating packaged product directly, one must account for the fact that forklift traffic and high-risk sanitation practices such as the use of pressure washers and compressed air can spread LM throughout a facility.
“Quoted content” above is from Strategies for Listeria Control in Tree Fruit Packing Houses, First Edition, United Fresh Produce Association, 2018.
Comments? Questions? Head on over to the forum to discuss.