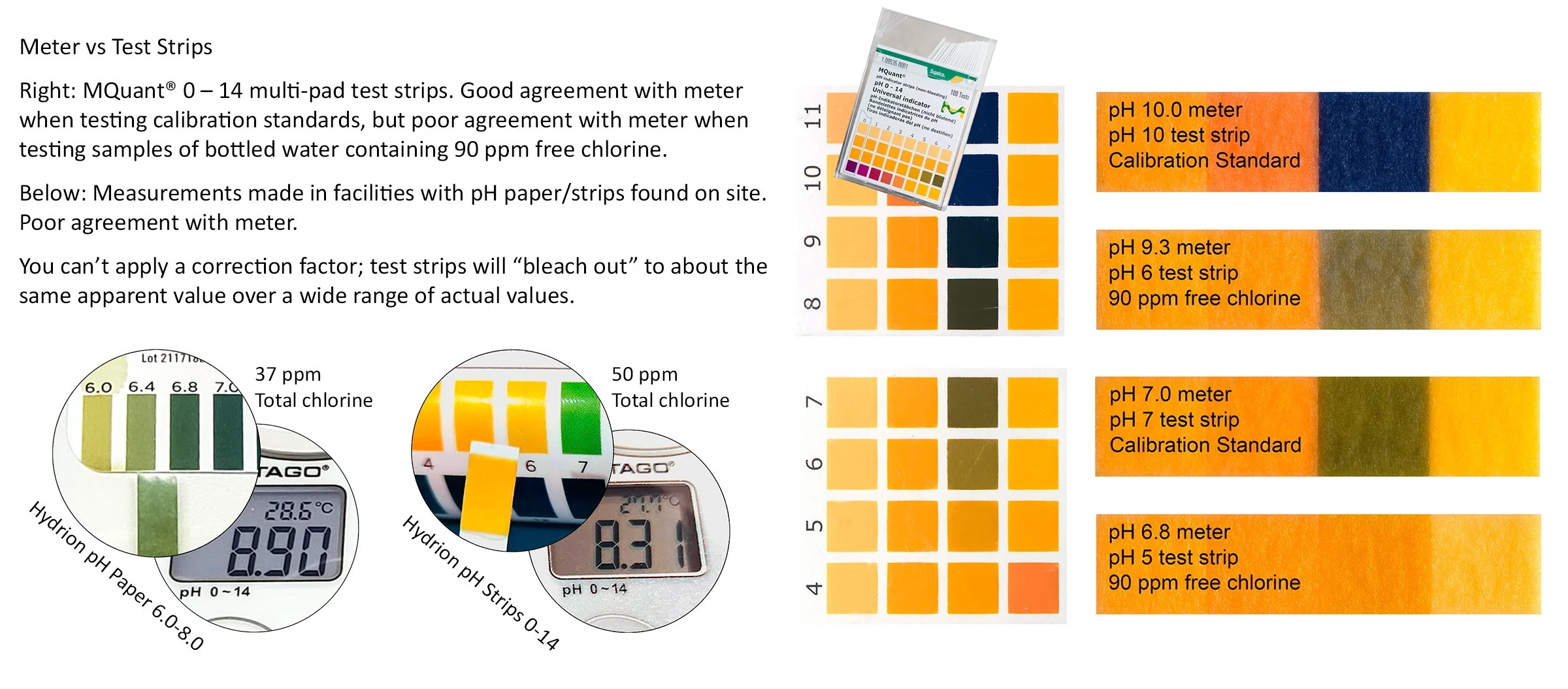
Throw away your pH test strips now!
Accurate monitoring and control of pH is critical for getting the most sanitizing “power” and bang for your buck out of sodium hypochlorite (chlorine bleach) solutions.
Test strips may be “accurate enough” when measuring the pH of drinking water or pool water containing very low concentrations of chlorine (less than 5 ppm). However, the concentration of sodium hypochlorite used in hydrocoolers and wash systems is usually great enough to cause chlorine to react with test strip dyes and change their color response to produce a much lower apparent pH value compared to actual. You may think the pH is 7, or even less, when it is actually 8 or 9.
Incorrect measurements may be worse than no measurements at all because they can give one a false sense that everything is okay when it is not. More than once, I have seen test strip results used as an argument for not controlling pH with an acidifying agent when actual pH was clearly too high as measured with an accurate, properly calibrated meter.
Sodium hypochlorite decreases from 80% active ingredient to 30% active ingredient as pH increases from 7 to 8. At pH 8, that’s like paying $13/gal instead of $5/gal for bleach. Good monitoring and control of pH pays for itself.
For most systems, a pH range of 6.5-7.5 on paper should accommodate normal system variation, but to the extent you can, it is best to maintain the pH of sodium hypochlorite solutions as close to 6.8-7.0 as possible.
NEVER USE TEST STRIPS TO MEASURE THE pH OF CHLORINATED HYDROCOOL OR WASH WATER.